Abstract
An increase in temperature increases the rate of chemical reactions. This is attributed to the rapid movement of molecules due to increased kinetic energy. This report focused on the effect of temperature on enzyme amylase. It was observed that the amylase activity increased with the rise in temperature from 4oC to 22oC, but declined with a further increase in temperature (80oC). These results were contrary to the expected results. The experiment was carried out under different temperature conditions to determine the influence of temperature on each reaction. A plot of temperature (x axis) against the rate of reaction (y axis) was made to determine the actual relationship between temperature and rate of reaction. This paper concluded that amylase reached its peak activity at 22oC (optimum temperature) and was denatured at a temperature of 80oC.
Introduction
Amylase is a vital enzyme in the human body because it aids in the digestion of starch. Amylase, like all enzymes, is a protein in nature and catalyzes biological reactions without getting consumed by the reactions. Diverse dynamics including temperature, pH, or trauma to the body of a human being disturb the reaction tempo of enzymes. According to Takai, Yamaguchi, Aragaki, Uchihashi, and Nishikawa (2004), stress in the human body regulates the release of amylase enzyme thus determining the reaction rate. Overall, a temperature increase by an interval of 10oC augments enzyme activity three times. Through the process of hydrolysis, the amylase enzyme breaks down starch into smaller and less complex glucose chains. The reaction produces a complex mixture that is composed mainly of maltose and glucose.
The focal point of this experiment was to ascertain the effect of temperature on the activity of amylase as a catalyst. Escalation in temperature yielded an increased activity of molecules because the molecules had additional kinetic energy. This energy increased the interaction of enzymes with substrates. In this experiment, a starch and glucose mixture was used as a substrate. The research question was whether a rise in temperature resulted in a linear increase in reaction rate. Therefore, the research hypothesis stated that the higher the temperature, the higher the speed of reaction. This experiment predicted that an increase in temperature shortened the reaction time.
Materials and Methods
Eight standard-sized test tubes were used in this experiment. The first four test tubes were labeled 1 to 4. Five milliliters of a 1% starch solution were added to the four test tubes using a calibrated pipette. 4ml of DI water and 1ml of 6.8 buffer were then added to every tube. Different conditions were provided for each test tube by placing them in water baths at different temperatures. The first test-tube was put inside a water bath at 80oC, second test-tube in a water bath at 37oC, the third test-tube in a test-tube rack at room temperature of about 22oC and the last test-tube was set on crushed ice at 4oC. A similar setup (standards) was done using the already mentioned steps using amylase instead of starch solution. This second set of test-tubes was labeled 1A to 4A. The two sets of test-tubes were incubated at the specified temperatures for 10 minutes.
Several rows of test plates were filled with two drops of I2KI per compartment. The contents of test-tube 1 and 1A were mixed and the time was recorded (time 0). In the same instance, two drops of the mixture (1 and 1A) were added to the test compartments containing the I2KI and the wells were observed for color changes.
New droplets of the mixture were added to the wells after every 30 seconds awaiting the disappearance of the blue coloration and appearance of a yellow-amber color. This was an indication that starch was fully digested. The experiment was stopped for each well when no color change was observed for the first 10 minutes. All the observations were recorded.
The steps indicated above were replicated for the other three sets of test-tubes. Changes and duration of those reactions were also recorded. For more advanced results, the test-tube on ice was transferred into the water bath at 37oC and incubated for about 2 minutes. The contents of the test-tube were then tested on test compartments containing I2KI at intervals of 10 seconds. These results were also recorded.
Results
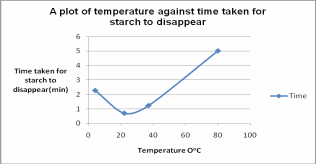
The values obtained were tabulated as shown in the below figure.
Table 1: Time of starch disappearance in Different temperatures for the enzyme amylase. The time taken in digestion starch was recorded in minutes. The temperature at which each reaction took place was also noted down.
In tube 1, there was no change even after 5 minutes of the experiment. In the second tube, the color changed from blue to yellow-amber after 1.22 minutes. The third tube recorded the shortest time in the experiment as the blue color disappeared after only 0.69 minutes. The last tube, which was placed on crushed ice, was recorded 2.26 minutes before the color change was observed.
Discussion
The results of this paper were contrary to the expected results. Although it was observed that an increase in temperature resulted in an increased reaction rate, this increase was not linear as expected. The water bath at 80oC recorded no change even after 5 minutes of the experiment. Extremely high and exceptionally low temperatures slowed down the reaction resulting in a long time before the color change was observed. Higher temperatures denatured the enzymes resulting in a slow rate of reaction or no reaction at all.
From the experiment, it was observed that amylase enzyme activity slowed down between 37oC and 80oC. There was no activity totally at 80oC because the high temperatures fully denatured the enzymes. Denatured enzymes showed no enzyme-substrate reaction hence no digestion of starch. Denaturation “depletes the system of active native enzyme hence apparently slows down the enzyme-substrate reaction” (Schneyer, 1950 p. 130). Protein denaturation entailed the destruction of chemical linkages in the molecular configuration of enzymes. Chemical bonds such as the sulphide and hydrogen bonds were upset by heat. Such bonds were vital for enzyme activity. That was why the enzyme did not act on the substrate at 80oC. Previous studies by Schneyer (1950) showed that enzyme activity could be restored after heating the enzyme for about 15 minutes at temperatures above the optimum. This experiment did not attempt to reestablish the activity of the enzymes that were denatured at 80oC.
However, when the test-tube placed on crushed ice was transferred to a water bath at 37oC, the reaction rate was increased. That was because the low temperatures did not alter the structure of enzymes. The low temperatures only made the enzymes become inactive. Therefore, when the temperature was altered, the motion of the enzymes was reinstated and thus increased the rate of the reaction (Takai et al., 2004). This observation also agreed with Schneyer that it was possible to revive enzyme activity after the denaturation process.
It was observed that the reaction time at 22oC was shorter than the reaction time at 37oC. This indicated that the optimum temperature for amylase was 22oC. However, a previous study obtained a range of 37oC to 45oC as the optimum temperature for amylase (Bailey, 1958). This temperature deviation was probably caused by errors in the experiment such as uncontrolled room temperature (room temperature was assumed to be 22oC, this was probably inaccurate). Poor handling of the test tubes and inaccuracies in timing also interfered with the results.
There was no enzyme activity shown at temperatures of 80oC. However, the exact point at which enzyme activity ceased was not determined as there was a wide range between the two temperature intervals (37oC and 80oC).
This study, therefore, concluded that amylase worked optimally at a temperature of 22oC. However, further research was needed to determine the actual temperature at which amylase was denatured.
References
Bailey, R. W. (1958). Bloat in cattle. The carbohydrases of the cattle rumen ciliate Epidinium ecaudatum Crawley isolated from cows fed on red clover (Trifolium pratense L.). New Zealand Journal of Crop and Horticultural Science/Experimental Agriculture, 1(3), 825-833.
Schneyer, L. H. (1950). The effect of temperature changes on salivary amylase activity. Journal of Dental Research, 30(1), 130-138. Web.
Takai, N., Yamaguchi, M., Aragaki, T., Eto, K., Uchihashi, K. & Nishikawa, Y. (2004). Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Archives of Oral Biology (49), 963-968. Web.

